DHR-ICMR Advanced Molecular Oncology Diagnostic Services (DIAMOnDS)
INTRODUCTION
DHR-ICMR Advanced Molecular Oncology Diagnostic Services (DIAMOnDS) is a sub-scheme established under the umbrella scheme of Human Resource and Capacity Development approved for the 15th Finance Commission period 2020-21 to 2025. This initiative aims to set up zonal oncopathology labs to provide basic as well as high-end advance diagnostic services to cancer patients and research facilities for basic, translational and clinical research.
The Department of Health Research initiated the DIAMOnDS as a pilot project in 2019, and implementing it as a Scheme since 2021-22, which is focussed on establishing labs for providing free of cost advanced molecular oncopathology diagnostic services for lung and breast cancers to the poor and needy patients. Till now, 24 DIAMOnDS centres have been established including one data management centre at ICMR-NCDIR (Bengaluru) and one quality assessment centre at ICMR-NIOP (Delhi). Moreover, the SFC has approved the establishment of 3 new DIAMOnDS centres every year till 2025-26, to ensure the geographical spread of the services and to bridge the gap in the infrastructure, ultimately improving the overall health status of the population.
OBJECTIVES
DIAMOnDS project aims to set up oncopathlogy labs to provide free of cost basic as well as high-end advance molecular pathology diagnostic services to cancer patients and research facilities for basic, translational and clinical research. These laboratories are established in Medical Institutes to ensure the optimum utilization of facilities available there, in terms of equipment and manpower and will also provide the much required diagnostic services to the cancer patients in those areas.
IMPLEMENTATION PLAN
A two-phase pilot development model has been adopted for the establishment of the DIAMOnDS centers. The process followed is as below:
Phase 1:
Step 1: Identify the institutes in high cancer incidence areas in India to setup oncopathology labs.
Step 2: Providing facilities, infrastructure and manpower to perform diagnostic and prognostic tests related to the cancer of highest prevalence in India in male (lung cancer) and female (breast cancer).
Step 3: Establishing and standardizing the tests related to Breast and lung cancer and developing a fast, digital system for reporting.
Phase 2:
Step 4: Creating awareness among the physicians, diagnostic service providers, primary health workers and patients about the facilities available at zonal oncopathology labs.
Step 5: Networking between the health and wellness centres, primary and community healthcare centres, districts hospitals, and medical colleges for proper guided referral.
Step 6: Monitoring and evaluation of services for assessment and improvements.
For further details, please refer to the DIAMOnDS guidelines here.
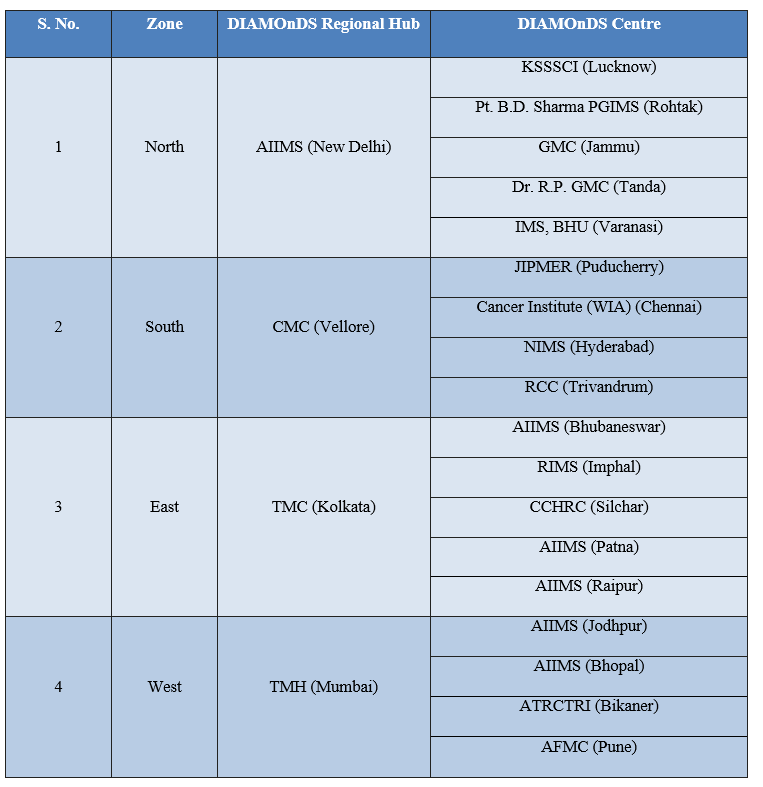
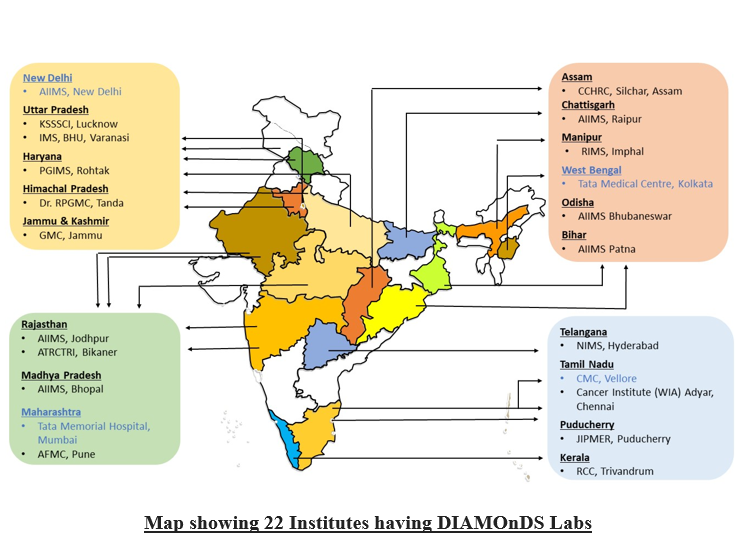
DIAMOnDS Hubs and centres
A total of 24 institutes including 4 institutes for establishing DIAMOnDS Hubs, 18 for establishing DIAMOnDS Centres, one institute (NCDIR) for data management, and one institute (NIOP Delhi) for Quality Assessment has been provided funds from the DIAMOnDS project.
Monitoring of DIAMOnDS Hubs/Centres
The evaluation of the services being offered by the DIAMOnDS Hubs and centres are regularly performed for assessment and improvements. Moreover, feedbacks from all the centres are also received in order to mitigate the challenges and issues being faced by the centres so as to provide hassle free services to the lung and breast cancer patients. To achieve this, DIAMOnDS centre visits are also undertaken by the DHR for elaborate discussion on key points with the Institute Director and the PI.
ROLES & RESPONSIBILITIES
DIAMOnDS Hubs
The following are the responsibilities of DIAMOnDS Hubs but not restricted to:-
1. To undertake cancer research studies independently or in collaboration with DHR and/or DIAMOnDS Hubs/centres. 2. Will provide support and guidance to the linked DIAMOnDS centres for the establishment of DIAMOnDS Labs, if required. Further, the Hubs PI may visit the linked centres regarding the same. 3. To monitor the linked DIAMOnDS centres to ensure optimum performance, standardization and proper testing of the cancer samples. 4. All DIAMOnDS Hubs will submit the cancer patient data to the ICMR-NCDIR Bengaluru that is linked to the National Cancer Registry Programme. 5. To participate in the quality assessment activities being undertaken by the ICMR-NIOP Delhi. 6. To organize and conduct training, workshop, etc. for DIAMOnDS staff and others in the field of molecular pathology/techniques. 7. To create awareness among the physicians, patients, primary and community health centres, district hospitals, medical colleges, etc. about the facilities available at DIAMOnDS labs for proper guided referral of the cancer patients. 8. Any other related task as assigned by DHR from time to time.
DIAMOnDS Centres
The following are the responsibilities of DIAMOnDS centres but not restricted to:-
1. To undertake cancer research studies independently or in collaboration with DHR and/or DIAMOnDS Hubs/centres. 2. To coordinate with the DIAMOnDS Hub in developing research proposals and generating grants. 3. To establish DIAMOnDS lab for performing immunohistochemistry and molecular pathology tests for cancer samples. 4. All DIAMOnDS centres will submit the cancer patient data to the ICMR-NCDIR Bengaluru that is linked to the National Cancer Registry Programme. 5. To participate in the quality assessment activities being undertaken by the ICMR-NIOP Delhi. 6. To create awareness among the physicians, patients, primary and community health centres, district hospitals, medical colleges, etc. about the facilities available at DIAMOnDS labs for proper guided referral of the cancer patients. 7. Any other related task as assigned by DHR from time to time.
ICMR-NIOP Delhi (Quality Assessment Centre)
The following are the responsibilities of NIOP for the DIAMOnDS project but not restricted to:-
1. To undertake cancer research studies independently or in collaboration with DHR and/or DIAMOnDS Hubs/centres 2. To constitute a Scientific Advisory Committee at the Institutional level for providing guidance and monitoring the progress regarding the quality assessment activities. 3. To perform quality assessment regarding the cancer diagnostic services provided by the DIAMOnDS Hubs and centres. 4. To send blinded samples (cancer, control, etc.) to the Hubs and centres, and assess their testing performance. 5. To organize and conduct training, workshop, etc. for DIAMOnDS staff and others in the field of molecular pathology/techniques. 6. Maintain all records related to the quality assessment, sample testing, fund utilization, etc. and should be submitted as and when requested by DHR. 7. Any other related task as assigned by DHR from time to time.
ICMR-NCDIR Bengaluru (Data Management Centre)
The following are the responsibilities of NCDIR for the DIAMOnDS project but not restricted to:-
1. To assist DIAMOnDS Hubs and centres in linking with the existing cancer registries to gather the data on cancer epidemiology. 2. To assist and provide training to DIAMOnDS Hubs and centres for cancer registry data entry in to the dedicated software. 3. To collect, collate, analyze and share the final data with DHR on monthly basis. 4. Any other related task as assigned by DHR from time to time.
Other Responsibilities of all centres
1. Memorandum of Agreement (MoA): A bilateral Memorandum of Agreement (MOA) will be signed by Institution and Government of India regarding the DIAMOnDS scheme. 2. The Institute may constitute a Scientific Advisory Committee for providing guidance and monitoring the progress of the DIAMOnDS lab. 3. DIAMOnDS grants to be placed in an Interest-bearing bank account maintained by the institute. The interest earned on these funds should be refunded to DHR. 4. To submit annual progress report to DHR. 5. Submission of Utilization certificate and Statement of Expenditure to DHR. 6. The DHR officials may visit the DIAMOnDS facility to monitor and evaluate their performance for assessments and improvements.
ACHIEVEMENTS
1. During FY 2024-25, four new DIAMOnDS centres were established, viz. All India Institute of Medical Sciences, Raipur (Chattisgarh); Institute of Medical Sciences, Banaras Hindu University, Varanasi (U.P.); Acharya Tulsi Regional Cancer Treatment and Research Institute, Bikaner (Rajasthan); and Armed Forces Medical College, Pune (Maharashtra). 2. The total number of patients benefitted by the scheme have been more than 15,000 (as on 14.10.2024), and the number of biomarker tests have crossed 75,000. 3. In terms of capacity building, a total of 308 people have been trained in the field of molecular pathology, and 95 in data management. 4. Three postdoctoral fellowship courses in the field of molecular pathology and cytogenetics are also being offered by the DIAMOnDS Hubs established at Mumbai, Vellore, and Kolkata. 5. The scheme currently provides employment to 89 people, and recruitment to some of the other posts are currently under process. 6. Under this scheme, 33 workshops and training programs have also been organized and conducted, and coordinated by the HTAIn division of DHR. 7. In addition, DIAMOnDS awareness and networking with other neighbouring institutes and the State Health Govt. officials is also being carried out by the DIAMOnDS centres, and is coordinated by the HTAIn division of DHR. 8. A total of 160 research papers have been published in various National and International journals. The establishment of the DIAMOnDS is also providing support to several research projects (both intramural and extramural) and M.D./Ph.D. theses work that are utilizing the DIAMOnDS laboratory facilities.
DIAMOnDS Labs
AIIMS, New Delhi


Cancer Institute WIA, Chennai




JIPMER, Puducherry


CMC, Vellore



AIIMS, Bhubaneswar


TMC, Kolkata








NIMS, Hyderabad




PGIMS Rohtak


AIIMS Jodhpur



RCC Thiruvananthapuram