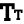National Ethics Committee Registry for Biomedical and Health Research (NECRBHR)
Department of Health Research
According to Chapter IV of the ‘New Drugs and Clinical Trials Rules 2019’, notified by The Ministry of Health and Family Welfare (Department of Health and Family Welfare), any organization conducting Biomedical and Health Research involving human participants, shall be required to have an ethics committee to review and oversee and conduct of such research as detailed in the 'National Ethical Guidelines for Biomedical and Health Research Involving Human Participants', specified by the Indian Council of Medical Research (ICMR).
In compliance with the sub-rule (l) of rule 17, the authority has been designated by the Department of Health Research (DHR) for registration of Ethics Committees for biomedical and health research involving human participants. Also, the 'National Ethics Committee Registry for Biomedical and Health Research' (NECRBHR), has been set up within the Department of Health Research to facilitate receipt and processing of applications seeking registration. It is now a mandatory requirement for those ethics committees intending to conduct biomedical and health research, involving human participants, to get registered with the designated authority in the DHR.
The entire process of receipt and processing of the applications, seeking registration of ethics committees for biomedical and health research involving human participants, shall be carried out online in the Department of Health Research via the NAITIK portal. All entities/institutions/medical colleges/researchers/stakeholders, etc. - whether in public or private and, who are conducting or intending to conduct biomedical and health research involving human participants - are required to register their Ethics Committees on the NAITIK portal.