Background
The development of evidence-based guidelines is crucial for enhancing healthcare quality, optimizing treatment strategies, and ensuring the efficient use of resources. Globally, organizations like the World Health Organization (WHO) continuously refine these guidelines through rigorous research and expert consensus. In India, the need for such guidelines is particularly pressing due to diverse healthcare challenges, regional disparities, and resource limitations. The fragmented nature of guideline development, along with the absence of a centralized authority, has impacted the uniformity and scientific rigor of clinical standards. The National Health Policy 2017 emphasized the importance of standardized, evidence-based guidelines applicable to both public and private healthcare sectors. The Department of Health Research (DHR) under the Department of Health and Family Welfare (DoHFW) initiated the Centre for Evidence-Based Guidelines (CEG) in February 2023. The CEG is mandated to systematically review available evidence, apply the GRADE methodology, and develop robust guidelines. It also engages in training sessions and workshops to enhance expertise in systematic reviews and evidence synthesis. In September 2024, the Centre launched 27 Technical Resource Centers (TRCs). Five specialized research hubs were also established to promote interdisciplinary collaboration and evidence-based decision-making.
Vision
• To improve healthcare quality in India. • To lead efforts in gathering and coordinating healthcare research. • To make scientifically backed guidelines accessible to healthcare professionals.
Mission
• The Centre is committed to coordinating the development of evidence-based guidelines that reflect the best available scientific research. • It aims to ensure these guidelines are aligned with national health priorities and India’s specific healthcare needs. • It seeks collaboration with DoHFW, NHSRC, ICMR, and other health agencies.
Objectives
• To formulate evidence-based clinical and public health guidelines. • To perform evidence synthesis for drafting clinical guidelines in line with the national priorities. • To improve healthcare delivery by providing consistent guidelines. • To collaborate with stakeholders for widespread adoption of guidelines. • To offer training and build capacity in systematic reviews and meta-analysis. • To establish a process for periodic update of guidelines.
List of Technical Resource Centers (TRCs)
1. Department of Laboratory Medicine, Rajendra Institute of Medical Sciences, Ranchi, Jharkhand. 2. Department of Pediatric Surgery, All India Institute of Medical Sciences, New Delhi. 3. Department of Pharmacy Practices, National Institute of Pharmaceutical Education and Research (NIPER), Mohali, Punjab. 4. Department of Orthopedics, Postgraduate Institute of Medical Education and Research, Chandigarh. 5. Department of Pharmacology, All India Institute of Medical Sciences, Jodhpur, Rajasthan. 6. Department of Epidemiology and Clinical Research, Institute of Liver and Biliary Sciences, New Delhi. 7. Department of Pharmacology, All India Institute of Medical Sciences Gorakhpur, Uttar Pradesh. 8. Division of Medical Research, SRM Medical College Hospital and Research Centre, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu. 9. ICMR-Centre for Ageing and Mental Health, Kolkata, West Bengal. 10 .Centre for Dental Education and Research, AIIMS, New Delhi. 11. ICMR-National Institute for Research in Tuberculosis, Chennai, Tamil Nadu. 12. Department of Infectious Diseases, Christian Medical College, Vellore, Tamil Nadu. 13. Department of Prosthodontics, King George's Medical University, Lucknow, Uttar Pradesh. 14. Department of Pediatrics, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh. 15. Department of Public Health, MS Ramaiah University of Applied Sciences, Karnataka. 16. Department of Biochemistry, All India Institute of Medical Sciences, Deoghar, Jharkhand. 17. Faculty of Nursing, Sri Ramachandra Institute of Higher Education and Research (Deemed to be University), Porur, Chennai, Tamil Nadu. 18. Department of Pediatric and Preventive Dentistry, Sri Ramchandra Institute of Higher Education and Research, Porur, Chennai, Tamil Nadu. 19. Department of Community Medicine, All Indian Institute of Medical Sciences, Nagpur, Maharashtra. 20. Department of Palliative Medicine and Supportive Care, KMC, MAHE, Manipal, Karnataka. 21. Department of Pharmacology, All India Institute of Medical Sciences, Guwahati, Assam. 22. Department of Ophthalmology, All India Institute of Medical Sciences, Guwahati, Assam. 23. School of Public Health, Postgraduate Institute of Medical Education and Research, Chandigarh, Punjab. 24. Department of Neonatology, Postgraduate Institute of Medical Education and Research, Chandigarh. 25. Department of Pharmacy Practice, Manipal College of Pharmaceutical Sciences, Karnataka. 26. Department of Public Health, Kalyan Singh Super Specialty Cancer Institute, Lucknow, Uttar Pradesh. 27. ICMR-National Institute for Research in Tuberculosis, Chennai, Tamil Nadu.
List of Technical Resource Hubs (TRHs)
1. Department of Paediatric Pulmonology, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, Punjab. 2. Department of Neurology, All India Institute of Medical Sciences (AIIMS), New Delhi. 3. Department of Reproductive Medicine and Surgery, Christian Medical College, Vellore. 4. Division of Evidence Synthesis, Datta Meghe Institute of Higher Education and Research (DMIHER), Wardha, Maharashtra. 5. Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research (NIPER), Guwahati, Assam. 6. Department of Surgical Oncology, Tata Memorial centre, Mumbai.
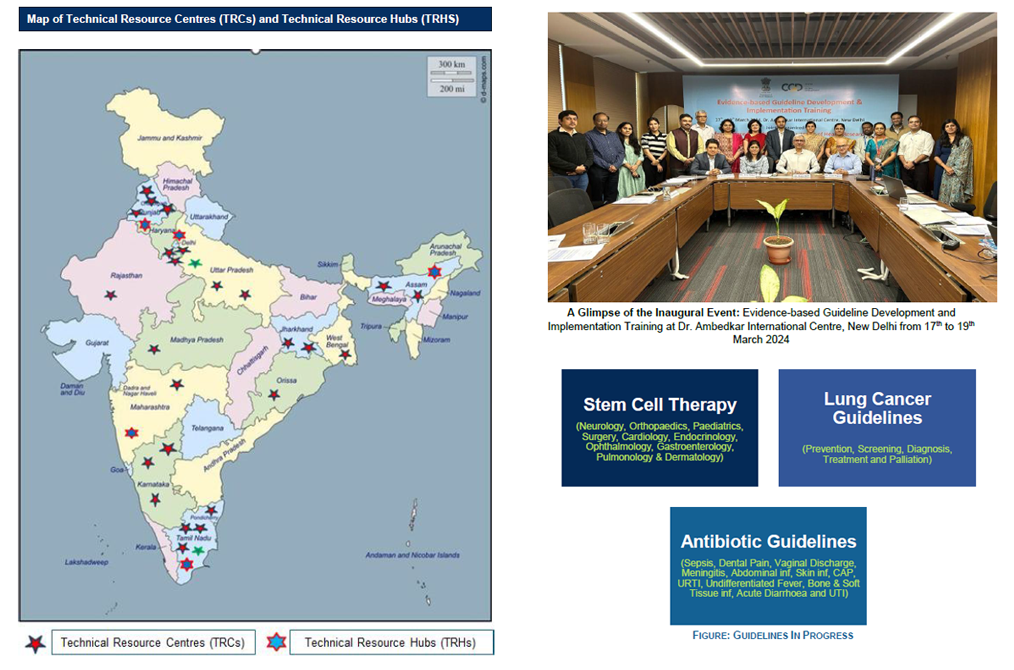
RELEASE OF EVIDENCE-BASED GUIDELINES FOR THE USE OF STEM CELL THERAPY
The Evidence based guidelines for the use of stem cell therapy in Neurological, Orthopedic, Pediatric and Cardiac disease conditions have been updated by adding a subgroup analysis based on the level of manipulation of stem cells in the included studies. In view of the update, the previous guidelines in the above four conditions stand null and void. The level of manipulation done to develop stem cell and stem cell derived products was interpreted by DHR secretariat into less than or more than minimal manipulation as defined by CDSCO (Annexed in guidelines) and the information provided in the trial itself.
| 1. | Evidence-based Guidelines for the use of Stem Cell Therapy in Neurological Conditions : Guidelines | Download |
|---|---|---|
| 2. | Evidence-based Guidelines for the use of Stem Cell Therapy in Neurological Conditions : Supplement | Download |
| 3. | Evidence-based Guidelines for the use of Stem Cell Therapy in Orthopedic Conditions : Guidelines | Download |
| 4. | Evidence-based Guidelines for the use of Stem Cell Therapy in Orthopedic Conditions : Supplement | Download |
| 5. | Evidence-based Guidelines for the use of Stem Cell Therapy in Pediatric Conditions : Guidelines | Download |
| 6. | Evidence-based Guidelines for the use of Stem Cell Therapy in Pediatric Conditions : Supplement | Download |
| 7. | Evidence-based Guidelines for the use of Stem Cell Therapy in Cardiac Conditions : Guidelines | Download |
| 8. | Evidence-based Guidelines for the use of Stem Cell Therapy in Cardiac Conditions : Supplement | Download |
| 9. | Evidence-based Guidelines for the use of Stem Cell Therapy in Endocrinological Conditions : Guidelines | Download |
| 10. | Evidence-based Guidelines for the use of Stem Cell Therapy in Endocrinological Conditions : Supplement | Download |
| 11. | Evidence-based Guidelines for the use of Stem Cell Therapy in Respiratory Conditions : Guidelines | Download |
| 12. | Evidence-based Guidelines for the use of Stem Cell Therapy in Respiratory Conditions : Supplement | Download |
On the Eve of World Cancer Day Honorable Union Health Minister Releases Evidence Based Guidelines for Lung Cancer Treatment and Palliation (3 February 2026)
The Department of Health Research today marked a significant milestone in India’s cancer care journey with the official release of the National Evidence-Based Guidelines for Lung Cancer Treatment and Palliation by the Hon’ble Union Minister for Health & Family Welfare, Shri Jagat Prakash Nadda. Developed through a rigorous, transparent, and multidisciplinary process, these guidelines provide 15 key evidence-informed recommendations focused on the management and palliation of lung cancer, tailored to the Indian healthcare context. The guidelines aim to promote standardized, high-quality clinical care, reduce unwarranted variations in practice, and strengthen decision-making across levels of healthcare delivery. This initiative reflects the Government of India’s continued commitment to improving cancer outcomes and integrating evidence-based approaches into national health policy and clinical practice.
The Evidence-Based Guidelines for Lung Cancer Treatment and Palliation can be accessed below:
| 1. | Evidence-Based Guidelines for Lung Cancer Treatment – Guidelines (12 recommendations) | Download |
|---|---|---|
| 2. | Evidence-Based Guidelines for Lung Cancer Treatment – Supplement | Download |
| 3. | Evidence-Based Guidelines for Lung Cancer Palliation – Guidelines (3 recommendations) | Download |
| 4. | Evidence-Based Guidelines for Lung Cancer Palliation – Supplement | Download |
Plain language summary for patients, caregivers and families can be accessed here
| 1. | Treatment options for Early-stage non-small cell Lung Cancer | Download |
|---|---|---|
| 2. | Treatment options for Advanced-stage non-small cell Lung Cancer | Download |
| 3. | Treatment options for small cell lung cancer | Download |
| 4. | Palliative care recommendations for patients with lung cancer | Download |
Workshops Organized
a) Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology workshop for systematic review teams of lung cancer guidelines was held on 1st August and 2nd August 2024 at DHR: •A two-day in-person workshop on the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) methodology was conducted on August 1–2, 2024, at the Department of Health Research (DHR). The primary objective was to provide comprehensive training to 25 systematic review teams involved in developing lung cancer guidelines. The workshop aimed to ensure uniform application of GRADE criteria across all systematic reviews, fostering consistency and methodological rigor in evaluating the evidence. •During the workshop, participants were introduced to the theoretical underpinnings of GRADE, including its core components such as assessing the certainty of evidence, grading recommendations, and understanding the evidence-to-decision framework. Extensive hands-on training sessions enabled participants to practically apply GRADE methodology to real-world datasets. b) Centre for guidelines scientists as resource persons for systematic review workshops: •Evidence-based Guideline Development using the GRADE Approach was held on 19-20 October 2024, at PGIMER Chandigarh organized by ICMR Advanced Centre for Evidence Based Child Health (ACEBCH) •Systematic review workshop for MPH scholars at ICMR-RMRC, Bhubaneswar, from 2nd to 5th April 2024 •Training cum workshop on Systematic Review and Meta-Analysis, held from 24 to 26 July 2024 at ICMR–RMRC, Gorakhpur. c)GRADE Methodology workshop for systematic review and meta-analysis conducted in Oct 2023. DHR Winter School workshop on Systematic Reviews & Meta-analysis conducted in Dec 2023. d)Training of 6 postgraduate interns (Master’s in Public Health) from All India Institute of Medical Sciences, Jodhpur (Rajasthan) in systematic reviews, evidence synthesis etc. apart from other activities conducted in DHR e.g. Bioethics Registry, Health Technology Assessment etc.

Systematic Reviews and Networking Support in Health (SARANSH)
•The SARANSH initiative was launched to strengthen regional capacity in conducting systematic reviews and meta-analyses across the country. This program provides funding support to medical institutes and colleges to organize high-quality, hands-on workshops focused on systematic reviews and meta-analysis methodologies. •A key objective of SARANSH is to foster collaborative networking between participating institutes/colleges and the DHR-Centre for Evidence-Based Guidelines. This partnership aims to enhance the quality and scope of systematic reviews, aligning them with evidence-based guideline development processes. •Ten SARANSH workshops have been successfully conducted in different regions of the country.
Workshops Conducted
| S No. | States/UT | Recommended Proposal | Institute/Medical College | Schedule |
|---|---|---|---|---|
| 1. | Gujarat & Rajasthan | Dr. Jaykaran Charan | All India Institute Of Medical Sciences, Jodhpur | 14-19 October 2024 |
| 2. | Maharashtra, Daman & Diu and Dadar & Nagar Haveli | Dr. Aravind Gandhi P | All India Institute Of Medical Sciences, Nagpur | 21-25 October 2024 |
| 3. | Madhya Pradesh, Chhattisgarh & Odisha | Dr. Amit Agrawal | All India Institute Of Medical Sciences, Bhopal | 12-16 November 2024 |
| 4. | Karnataka & Goa | Dr. Girish Thunga | Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal | 18-22 November 2024 |
| 5. | Tamil Nadu, Kerala, Puducherry & Andaman & Nicobar Islands | Dr. Chandrashekar Janakiram | Amrita School of Dentistry, Amrita Vishwa Vidyapeethan, Kochi | 2-7 December 2024 |
| 6. | Uttar Pradesh & Uttarakhand | Dr. Balendra Pratap Singh | King George Medical University, Lucknow | 4-7 December 2024 |
| 7. | Assam, Manipur,Tripura,Arunachal Pradesh,Meghalaya, Mizoram Naghalang & Sikkim | Dr. Krishna Undela | National Institute of Pharmaceutical Education and Research Guwahati | 9-13 December 2024 |
| 8. | Punjab,Jammu & Kashmir,Ladakh,Himachal Pradesh,Chandigarh, Haryana & Delhi | Dr. Sachit Anand | All India Institute of Medical Sciences, New Delhi | 18-22 December 2024 |
| 9. | West Bengal, Bihar & Jharkhand | Dr. Seshadri Reddy Varikasuvu | All India Institute of Medical Sciences, Deoghar | 20-23 January 2025 |
| 10. | Telangana & Andhra Pradesh | Dr. Sai Krishna Tikka | All India Institute of Medical Sciences, Bibinagar | 19-22 February 2025 |

Strategic Conclave for TRCs
• Convened in January 2025 at Mahabalipuram, Tamil Nadu. • Brought together Principal Investigators (PIs) from 27 TRCs to discuss collaboration, standardization, and share best practices.
CEG Scientists
| S No. | Name of the Scientist | Designation |
|---|---|---|
| 1. | Dr. Roopa Hariparsad | Scientist - F |
| 2. | Dr. Chanchal Goyal | Scientist - F |
| 3. | Dr. Vikas Dhikav | Scientist - E |
| 4. | Dr. Varsha Dalal | Scientist - E |
| 5. | Dr. Debjani Ram Purakayastha | Scientist - D |
| 6. | Dr. Siddharth Kapahtia | Scientist - D |
| 7. | Dr. Vikas Dhiman | Scientist - D |
Nodal Officials
| S No. | Name of the Officer | Designation | Email ID |
|---|---|---|---|
| 1. | Dr. Tushar Karmarkar | Deputy Secretary | tushar[dot]arun[at]gov[dot]in |
| 2 | Ms. Sreelatha A K | Under Secretary | sreelatha[dot]ak[at]nic[dot]in |
For any queries pertaining to Centre for Evidence-based Guideline, Please contact on centreforguidelines[at]gmail[dot]com